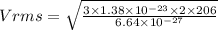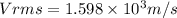Answer:
a)1.13×10³
b)1.6×10³
Step-by-step explanation:
Given:
Boltzmann's constant (K)=1.38×10^-23 J/K
atmoic mass of helium = 4 AMU or 4×1.66×10^-27kg
a)The formula for RMS speed (Vrms) is given as
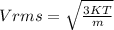
where
K= Boltzmann's constant
T= temperature
m=mass of the gas
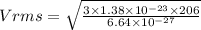
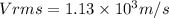
b) RMS speed of helium when the temperature is doubled