Given:
 = 50°C = 273+50 =323 K
= 50°C = 273+50 =323 K
 = 300°C = 273+300 =573 K
= 300°C = 273+300 =573 K
Solution:
We know that:
specific heat for gold, c = 0.129 J/g°C
Also, change in entropy, ΔS is given by:
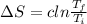
After the bars brought in contact with each other,
final temperature,
 =
=
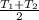
final temperature,
 =
=
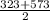 = 448K
= 448K
Now, entropy for first gold bar, using eqn-1
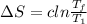
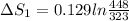 =0.042 J/K
=0.042 J/K
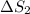 = 0.129ln
= 0.129ln
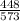 = - 0.032 J/K
= - 0.032 J/K
Total entropy generation,
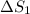 =
=
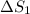 +
+
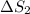
 = 0.042 + (- 0.032) = 0.010 J/K
= 0.042 + (- 0.032) = 0.010 J/K