Answer:
Here's what I get.
Step-by-step explanation:
The Antoine equation is
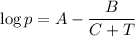
A = 13.7819
B = 2726.81
C = 217.572
I did the calculations and the plots in Excel.
Figure 1 shows the calculations, Figure 2 is the linear plot, and Figure 3 is the log plot.