Answer:
P2 = 778.05 mm Hg = 1.02 atm
Step-by-step explanation:
We are to find the final pressure (expressed in atm) of a 3.05 liter system initially at 724 mm hg and 298 K which is compressed to a final volume of 2.60 liter at 273 K.
For this, we would use the equation:
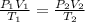
where P1 = 724 mm hg
V1 = 3.05 L
T1 = 298 K
P2 = ?
V2 = 2.6 L
T2 = 173 K
Substituting the given values in the equation to get:
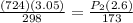
P2 = 778.05 mm Hg = 1.02 atm