Answer:
The pressure inside the cooker is 1.0804 atm.
Step-by-step explanation:
Boiling occurs when the vapor pressure becomes equal to atmospheric pressure.
For water, At standard conditions (Pressure = 1 atm) boiling occurs at 373.15 K.
So, Standard conditions:
T₁ = 373.15 K
P₁ = 1 atm
Given ,
The water boils at Temperature = 130 °C
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So, the temperature, T₂ = (130 + 273.15) K = 403.15 K
To find pressure inside the cooker (P₂) :
Applying Amontons's Law as:
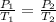
So,
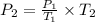
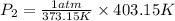
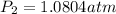
Thus, The pressure inside the cooker is 1.0804 atm.