Answer:
The pH of a solution containing 0.1 mM H+ is 4
Step-by-step explanation:
The pH of any solution is given by the following mathematical equation -
![pH = -log[H_(+)]\\](https://img.qammunity.org/2020/formulas/biology/college/w6qli2zipjl01g248uch803jwwdns5gma4.png) ----------- Equation (A)
----------- Equation (A)
Where,
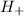 = the concentration of hydrogen ion in the solution.
= the concentration of hydrogen ion in the solution.
Given
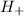
=
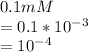
Substituting the given value in equation (A) , we get -
![pH = -log[10^(-4)]](https://img.qammunity.org/2020/formulas/biology/college/zjegoz98gx0skmtp0d96ug8enuo7aovdck.png)
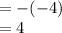
Hence, The pH of a solution containing 0.1 mM H+ is 4