Answer:
Minimum uncertainty in position is
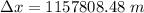
Step-by-step explanation:
It is given that,
Uncertainty in the position of an electron,
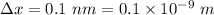
According to uncertainty principle,
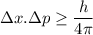


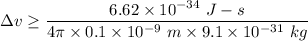
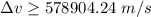
Let
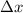 is the uncertainty in position after 2 seconds such that,
is the uncertainty in position after 2 seconds such that,
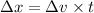
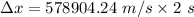
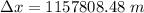
or
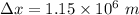
Hence, this is the required solution.