Answer: Yes
Step-by-step explanation:
Limiting reagent is the reagent which limits the formation of product as it gets completely consumed in the reaction.
Excess reagent is the reagent which is left unreacted in the reaction.
For example:
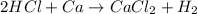
If there are 2 moles of
 and 2 moles of
and 2 moles of
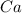
As can be seen from the chemical equation,
2 moles of hydrochloric acid react with 1 mole of calcium.
Thus 2 moles of
 will completely react with 1 mole of calcium and (2-1)=1 mole of calcium will remain as such.
will completely react with 1 mole of calcium and (2-1)=1 mole of calcium will remain as such.
Thus HCl is the limiting reagent as it limits the formation of product and calcium is the excess reagent as it is left unreacted.