Answer:
a). Work transfer = 527.2 kJ
b). Heat Transfer = 197.7 kJ
Step-by-step explanation:
Given:
 = 5 Mpa
= 5 Mpa
 = 1623°C
= 1623°C
= 1896 K
 = 0.05
= 0.05

Also given
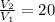
Therefore,
 = 1
= 1

R = 0.27 kJ / kg-K
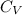 = 0.8 kJ / kg-K
= 0.8 kJ / kg-K
Also given :
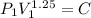
Therefore,
 =
=
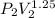
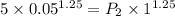
 = 0.1182 MPa
= 0.1182 MPa
a). Work transfer, δW =
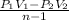
![\left [(5* 0.05-0.1182* 1)/(1.25-1) \right ]* 10^(6)](https://img.qammunity.org/2020/formulas/engineering/college/f53h1ji7oahtyom6blb5qj2ko1k7zc1fyc.png)
= 527200 J
= 527.200 kJ
b). From 1st law of thermodynamics,
Heat transfer, δQ = ΔU+δW
=
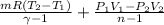
=
![\left [ (\gamma -n)/(\gamma -1) \right ]* \delta W](https://img.qammunity.org/2020/formulas/engineering/college/x8tye23h38gi9rmqadbrimouf89045akdu.png)
=
![\left [ (1.4 -1.25)/(1.4 -1) \right ]* 527.200](https://img.qammunity.org/2020/formulas/engineering/college/hwj1nui1fzr59l2b8k16i7epddu29zppwn.png)
= 197.7 kJ