Answer:
Here's what I get.
Step-by-step explanation:
The MO diagrams of KrBr, XeCl, and XeBr are shown below.
They are similar, except for the numbering of the valence shell orbitals.
Also, I have drawn the s and p orbitals at the same energy levels for both atoms in the compounds. That is obviously not the case.
However, the MO diagrams are approximately correct.
The ground state electron configuration of KrF is
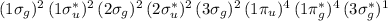
KrF⁺ will have one less electron than KrF.
You remove the antibonding electron from the highest energy orbital, so the bond order increases.
The KrF bond will be stronger.