Answer:
49.6mL
Explanation:
Given parameters:
Volume of solution = 386mL
Percentage composition of acid = 12.86%
Solution
The solution is made up of water and acid in their respective proportions.
We have been given the percentage composition of acid to be 12.86%, so we can easily obtain the exact volume of the acid in the compositon.
Volume of acid in solution = perenctage of acid x volume of solution
Volume of acid =
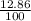 x 386mL = 49.6396mL
x 386mL = 49.6396mL
Rounding to the nearest tenth gives: 49.6mL