Answer: Strong electrolytes:
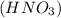 Nitric acid,
Nitric acid,
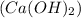 )Calcium Hydroxide and (KCl) potassium chloride
)Calcium Hydroxide and (KCl) potassium chloride
Weak electrolytes:
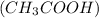 Acetic acid and
Acetic acid and
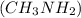 Methyl amine
Methyl amine
Non-electrolytes:
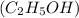 Ethanol and
Ethanol and
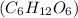 Glucose
Glucose
Step-by-step explanation: Electrolytes are those compounds which can conduct electricity when dissolved in any polar solvent.
Strong electrolytes are those compounds which completely ionise when dissolved in polar solvent and hence produce ions in solution . So greater the capacity of an compound to ionize itself greater number of ions would be present in solution and hence greater will be the capacity of the solution to conduct electricity.
Ionic compounds like
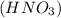 Nitric acid ,(KCl) Potassium chloride and
Nitric acid ,(KCl) Potassium chloride and
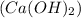 )Calcium hydroxide are completely ionized when dissolved in polar solvent so these compounds are strong electrolytes.
)Calcium hydroxide are completely ionized when dissolved in polar solvent so these compounds are strong electrolytes.
Weak electrolytes are those compounds which undergo partial ionization when dissolve in polar solvents . So they are not able to produce more ions in the solution and hence the conductivity of a solution containing weak electrolytes is low.
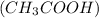 Acetic acid and
Acetic acid and
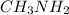 Methyl amine are partially ionized when dissolved in polar solvent so these electrolytes are weak electrolytes.
Methyl amine are partially ionized when dissolved in polar solvent so these electrolytes are weak electrolytes.
Non-electrolytes are those compounds which are not at all ionized in the polar solvent and they remain as molecules itself even if they are dissolved.
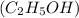 Ethanol and
Ethanol and
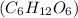 Glucose do not ionize when dissolved in polar solvent and remain as molecules itself so the solutions of these compounds will not have ions and hence they would be unable to conduct electricity.
Glucose do not ionize when dissolved in polar solvent and remain as molecules itself so the solutions of these compounds will not have ions and hence they would be unable to conduct electricity.
so