Solution:
Given:
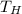 = 1200 K
= 1200 K
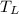 = 600 K
= 600 K
Q = 100 kJ
The Entropy change of the two reservoirs is given by the sum of entropy change of each reservoir system and is given by the formula:
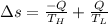
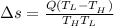
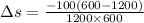
[tex]\Delta s = 0.0833kJ/K
Since, the change in entropy is positive and according to the Increase in entropy principle, for any process the total change in entropy of a system is always greater than or equal to zero (with its enclosing adiabatic surrounding).
Therefore, the entropy principle is satisfied.