Answer:
19.9 grams of
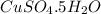 will be needed.
will be needed.
Step-by-step explanation:
Required strength of the solution = 2% (w/v)
This means that 2 gram of solute in 100 ml of solution.
Mass of
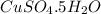 = 2 g
= 2 g
Moles of
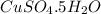 =
=
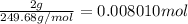
Volume of the solution = 100 mL = 0.1 L
Molarity of the solution:
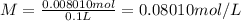
0.08010 moles of
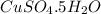 are present 1 l of the solution.
are present 1 l of the solution.
Then mass of 0.08010 moles of
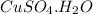 will be:
will be:
0.08010 mol × 249.68 g/mol = 19.9993 g≈ 19.9 g
19.9 grams of
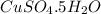 will be needed.
will be needed.