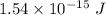Answer:
The kinetic energy of an electron is
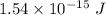
Step-by-step explanation:
Given that,
Distance = 0.1 nm
We need to calculate the momentum
Using uncertainty principle
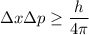
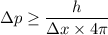
Where,
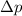 = change in momentum
= change in momentum
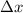 = change in position
= change in position
Put the value into the formula
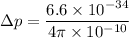
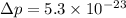
We need to calculate the kinetic energy for an electron
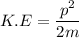
Where, P = momentum
m = mass of electron
Put the value into the formula
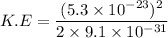
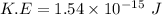
Hence, The kinetic energy of an electron is