Answer:
The w/w 5 of the solution is 10 %.
Step-by-step explanation:
w/w % : The percentage mass or fraction of mass of the of solute present in total mass of the solution.
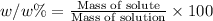
w/v %: The percentage of mass of the of solute present in total volume of the solution.
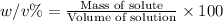
v/v % : The percentage volume of the of solute present in total volumeof the solution.
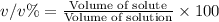
Mass of solution = Mass of solute + Mass of solvent
Mass of solute= 10 g of NaOH
Mass of solvent = 90 g
Mass of solution = 10 g + 90 g = 100 g
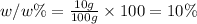
The w/w 5 of the solution is 10 %.