Answer:
The molar concentration of
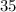 g fructose in
g fructose in
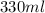 solution is
solution is
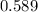 moles/litre
moles/litre
Step-by-step explanation:
Given -
Molecular weight of fructose
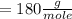
Can of coka coal has fructose
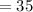 gram
gram
Volume of can of coke
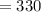 ml
ml
Number of moles in
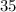 grams of Fructose
grams of Fructose
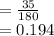
Molar concentration
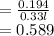 moles/litre
moles/litre
Hence, the molar concentration of
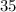 g fructose in
g fructose in
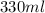 solution is
solution is
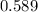 moles/litre
moles/litre