Answer:
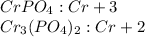
Step-by-step explanation:
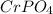 and
and
 are compounds known as ternary salts
are compounds known as ternary salts
This means that they are formed by a metal or a non-metal and an anion
Its formula is
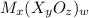 , that is, the cation is written first and then the anion and the simplified charges are exchanged if possible.
, that is, the cation is written first and then the anion and the simplified charges are exchanged if possible.
The anion
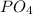 has a load of -3:
has a load of -3:
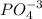
Let's look at the first compound
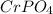 we observe that when exchanging the charges 3 of the
we observe that when exchanging the charges 3 of the
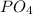 does not appear therefore the charges are simplified as the charges are completely simplified, it means that the chromium has the same valence (numerically but with opposite sign) that the anion
does not appear therefore the charges are simplified as the charges are completely simplified, it means that the chromium has the same valence (numerically but with opposite sign) that the anion
Therefore the oxidation state of Cr in
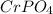 is +3
is +3
Let's look at the second compound
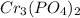 , it is observed that when exchanging the valences, the 3 of the
, it is observed that when exchanging the valences, the 3 of the
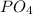 is with the chromium, and with the anion is 2
is with the chromium, and with the anion is 2
As valencia are not multiples, they cannot be simplified.
When exchanging the valences, the
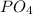 has the valence corresponding to the chromium which in this case is + 2
has the valence corresponding to the chromium which in this case is + 2