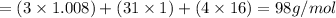Answer:
A. 837 grams of
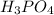 are produced when 12.81 moles of water react with an excess of
are produced when 12.81 moles of water react with an excess of
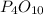
Step-by-step explanation:
The balanced chemical equation is
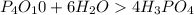
Mole ratio of
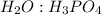 is 6 : 4 or 3 : 2
is 6 : 4 or 3 : 2
As per the Equation
6 moles of water produces 4 moles of Phosphoric acid
So let us convert
12.81 moles
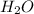 to moles
to moles
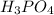 by using mole ratio and then to mass
by using mole ratio and then to mass
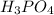 by multiplying with molar mass
by multiplying with molar mass
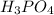
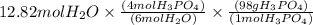
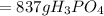 is produced
is produced
Please note:
Molar mass is the mass of 1 mole of the substance and its unit is g/mol
We find the molar mass by adding the atomic mass of the atoms present in it.
For example
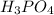 contains 3 atoms of H, 1 atom of P and 4 atoms of O
contains 3 atoms of H, 1 atom of P and 4 atoms of O
So the molar mass