Answer: 71.65 L
Step-by-step explanation:
Decomposition of sodium azide is shown by equation below:
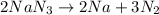

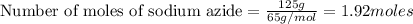
According to stoichiometry:
2 moles of
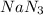 produce 3 moles of
produce 3 moles of

Thus 1.92 moles of
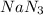 will produce=
will produce=
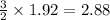 moles of
moles of

According to the ideal gas equation:

P = Pressure of the gas = 756 torr = 0.99 atm (1 torr= 0.0013 atm)
V= Volume of the gas = ?
T= Temperature of the gas = 27°C = 300 K (0°C = 273 K)
R= Gas constant = 0.0821 atmL/K mol
n= moles of gas= 2.88
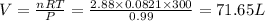
Thus the volume of nitrogen gas at 27 °C and 756 torr formed by the decomposition of 125 g of sodium azide is 71.65 L