Step-by-step explanation:
Electric force is the force acting between two charged particles. Electric force between electron and proton is given by :
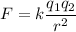
Distance between them,
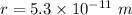
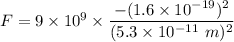
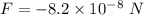
Gravitational force is the force acting between two masses. The gravitational force between the electron and proton is given by :
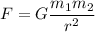
Distance between them,
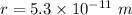
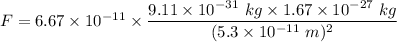

Hence, this is the required solution.