Answer:
Phosphoric acid
Step-by-step explanation:
Phosphoric acid is an oxyacid, which is formed when hydrogen combines with the polyatomic ion phosphate. The polyatomic ion for phosphate is
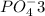 . To combine this with hydrogen, we "criss-cross" the charges of phosphate (which has a charge of -3) and hydrogen (which has a charge of +1) to get
. To combine this with hydrogen, we "criss-cross" the charges of phosphate (which has a charge of -3) and hydrogen (which has a charge of +1) to get
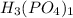 , the same as
, the same as
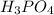 .
.
To answer this question, we essentially reverse these steps. First, we would recognize that this is a hydrogen atom bonded with phosphate. Because phosphate is a polyatomic ion, we know that this is an oxyacid. Because it involves phosphate, we call it phosphoric acid.
I hope this helps!