Answer: 0.028 grams
Step-by-step explanation:
Depression in freezing point :
Formula used for lowering in freezing point is,
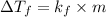
or,
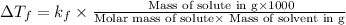
where,
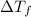 = change in freezing point
= change in freezing point
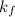 = freezing point constant (for benzene} =
= freezing point constant (for benzene} =
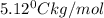
m = molality
Putting in the values we get:
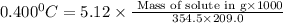
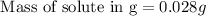
0.028 grams of DDT (solute) must be dissolved in 209.0 grams of benzene to reduce the freezing point by 0.400°C.