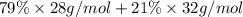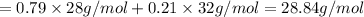Answer:
Average molecular weight of air is 28.84 g/mol.
Step-by-step explanation:
The average molecular weight of a mixture is determined from their molar composition and molecular weight.
Average molecular weight :
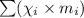
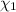 : mole fraction of the 'i' component.
: mole fraction of the 'i' component.
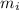 = Molecular weight of i component
= Molecular weight of i component
Average molecular weight of air with approximate molar composition of 79% nitrogen gas and 21% of oxygen gas can be calculated as:
Average molecular weight of air: