Step-by-step explanation:
The Coulomb's law states that the force acting on two charges is directly proportional to the product of charges and inversely proportional to the square of distance between them . Mathematically, it is given by
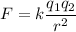
Where
k is the electrostatic constant
q₁ and q₂ are charges
r is the distance between them.
The SI unit of electric force is Newton. It can be attractive or repulsive. The attraction or repulsion depend on charges. If both charges are positive, the force is repulsive and if both are opposite charges, the force is attractive.