Answer:
(a)
 = -0.098 KJ/kg-K
= -0.098 KJ/kg-K
(b)P= 29.8 KW
(c)
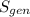 = -0.098 KW/K
= -0.098 KW/K
Step-by-step explanation:
 mass flow rate= 1 kg/s.
mass flow rate= 1 kg/s.
T=25°C
Air treating as ideal gas
(a)
We know that entropy change for ideal gas between two states
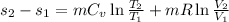
Given that this is isothermal process so
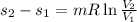
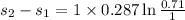
 = -0.098 KJ/kg-K
= -0.098 KJ/kg-K
(b)
Power required
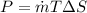
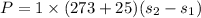
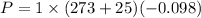
P= -29.8 KW (Negative sign means it is compression process.)
(c)
Rate of entropy generation
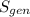
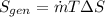
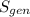 =1(-0.098)
=1(-0.098)
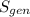 = -0.098 KW/K
= -0.098 KW/K