Answer : The number of faradays of electricity had to pass through the solution will be, 0.596 F
Explanation :
First we have to calculate the moles of oxygen gas.
using ideal gas equation:

where,
P = pressure of gas = 755 mm Hg = 0.99 atm
conversion used : (1 atm = 760 mmHg)
V = volume of gas = 3.696 L
T = temperature of gas =
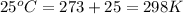
n = number of moles of gas = ?
R = gas constant = 0.0821 L.atm/mole.K
Now put all the given values in the ideal gas equation, we get the number of moles of oxygen gas.
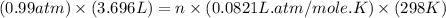
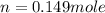
Now we have to calculate the number of faradays of electricity had to pass through the solution.
The balanced half-reactions for the electrolysis of water is,
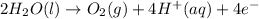
From this we conclude that,
As, 1 mole of oxygen gas require 4 mole of electrons that means 4 F (faraday) of electricity.
So, 0.149 mole of oxygen gas require
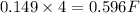 of electricity.
of electricity.
Therefore, the number of faradays of electricity had to pass through the solution will be, 0.596 F