Answer:
The pressure of the remaining gas in the tank is 6.4 atm.
Step-by-step explanation:
Given that,
Temperature T = 13+273=286 K
Pressure = 10.0 atm
We need to calculate the pressure of the remaining gas
Using equation of ideal gas

For a gas
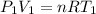
Where, P = pressure
V = volume
T = temperature
Put the value in the equation
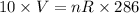 ....(I)
....(I)
When the temperature of the gas is increased
Then,
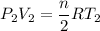 ....(II)
....(II)
Divided equation (I) by equation (II)
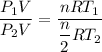
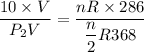
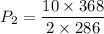
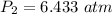
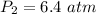
Hence, The pressure of the remaining gas in the tank is 6.4 atm.