Answer:
a)W=20 KJ
b) ΔQ= 220 KJ
Step-by-step explanation:
Given:
V₁=0.1 m^3, P₁=200 kPa and heat is added to the system such that system expands under constant pressure.
Therefore V₂= 2V₁= 0.2 m^3
a) Work transfer W= P(V₂-V₁)=
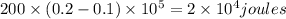
W=20 KJ
b) internal energy change ΔU= 200 KJ
from first law we know that ΔQ(net heat transfer)= ΔU + W
ΔQ=
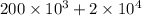
ΔQ=
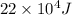
ΔQ= 220 KJ