Answer:
14.9802 grams of estrogen must be added to 216.7 grams of benzene.
Step-by-step explanation:
The relative lowering of vapor pressure of solution containing non volatile solute is equal to mole fraction of solute.
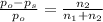
Where:
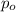 = Vapor pressure of pure solvent
= Vapor pressure of pure solvent
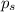 = Vapor pressure of the solution
= Vapor pressure of the solution
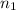 = Number of moles of solvent
= Number of moles of solvent
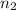 = Number of moles of solute
= Number of moles of solute
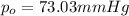
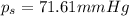
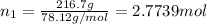
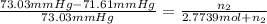
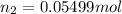
Mass of 0.05499 moles of estrogen :
= 0.05499 mol × 272.4 g/mol = 14.9802 g
14.9802 grams of estrogen must be added to 216.7 grams of benzene.