Step-by-step explanation:
It is given that,
Mass of lithium,
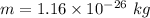
It is accelerated through a potential difference, V = 224 V
Uniform magnetic field, B = 0.724 T
Applying the conservation of energy as :
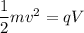
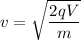
q is the charge on an electron
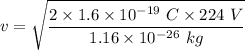
v = 78608.58 m/s

To find the radius of the ion's path in the magnetic field. The centripetal force is balanced by the magnetic force as :
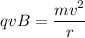
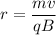
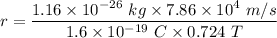
r = 0.0078 meters
So, the radius of the path of the ion is 0.0078 meters. Hence, this is the required solution.