Step-by-step explanation:
It is given that,
Momentum of the photon,
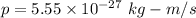
(a) We need to find the wavelength of this photon. It can be calculated using the concept of De-broglie wavelength.
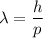
h is the Planck's constant
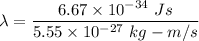
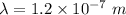
or
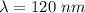
(b) The wavelength lies in the group of ultraviolet rays. The wavelength of UV rays lies in between 400 nm to 10 nm.