Answer : The heat of this reaction of AgCI formed will be, 66.88 KJ
Explanation :
First we have to calculate the heat of the reaction.
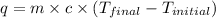
where,
q = amount of heat = ?
 = specific heat capacity =
= specific heat capacity =
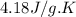
m = mass of substance = 120 g
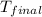 = final temperature =
= final temperature =
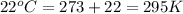
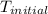 = initial temperature =
= initial temperature =
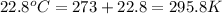
Now put all the given values in the above formula, we get:
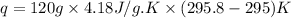
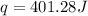
Now we have to calculate the number of moles of
 and
and
 .
.
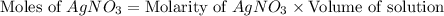
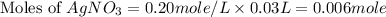
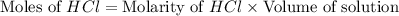
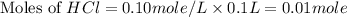
Now we have to calculate the limiting reactant.
The balanced chemical reaction will be,
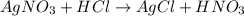
As, 1 mole of
 react with 1 mole of HCl
react with 1 mole of HCl
So, 0.006 mole of
 react with 0.006 mole of HCl
react with 0.006 mole of HCl
From this we conclude that,
 is an excess reagent because the given moles are greater than the required moles and
is an excess reagent because the given moles are greater than the required moles and
 is a limiting reagent and it limits the formation of product.
is a limiting reagent and it limits the formation of product.
Now we have to calculate the moles of AgCl.
The given balanced reaction is,
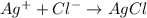
From this we conclude that,
1 mole of
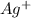 react with 1 mole
react with 1 mole
 to produce 1 mole of
to produce 1 mole of
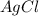
0.006 mole of
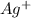 react with 0.006 mole
react with 0.006 mole
 to produce 0.006 mole of
to produce 0.006 mole of
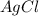
Now we have to calculate the heat of this reaction of AgCI formed.
As, 0.006 mole of AgCl produced the heat = 401.28 J
So, 1 mole of AgCl produced the heat =
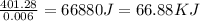
Therefore, the heat of this reaction of AgCI formed will be, 66.88 KJ