Answer : The time taken for the reaction is, 28 s.
Explanation :
Expression for rate law for first order kinetics is given by :
![k=(2.303)/(t)\log([A_o])/([A])](https://img.qammunity.org/2020/formulas/chemistry/college/67w3lufh8bppkbbcp1xaut7f9029vt6k0l.png)
where,
k = rate constant = 0.0632
t = time taken for the process = ?
![[A_o]](https://img.qammunity.org/2020/formulas/chemistry/high-school/38eb24kf04xqy5t88y9g0vzh3m04r4nqgg.png) = initial amount or concentration of the reactant = 1.28 M
= initial amount or concentration of the reactant = 1.28 M
![[A]](https://img.qammunity.org/2020/formulas/chemistry/college/ey5pxctwpmy356s81f6qc2pjl6oveugako.png) = amount or concentration left time 't' =
= amount or concentration left time 't' =
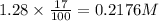
Now put all the given values in above equation, we get:
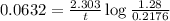
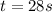
Therefore, the time taken for the reaction is, 28 s.