Answer:
Approximately 206 grams.
Step-by-step explanation:
How many moles of sulfuric acid
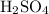 are there in this solution?
are there in this solution?
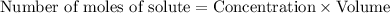 .
.
The unit for concentration "
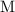 " is equivalent to mole per liter. In other words,
" is equivalent to mole per liter. In other words,
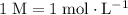 . For this solution, the concentration of
. For this solution, the concentration of
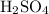 is
is
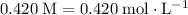 .
.
 .
.
What's the mass of that
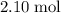 of
of
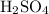 ?
?
Start by finding the molar mass
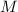 of
of
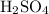 .
.
Relative atomic mass data from a modern periodic table:
- H: 1.008;
- S: 32.06;
- O: 15.999.
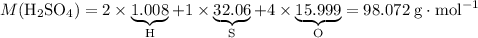 .
.
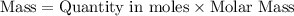 .
.
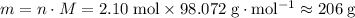 .
.
In other words, the chemist shall need approximately 206 grams of
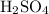 to make this solution. As a side note, keep in mind that the 206 grams of
to make this solution. As a side note, keep in mind that the 206 grams of
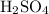 also take up considerable amount of space. Therefore it will take less than 5.00 L of water to make the 5.00 L solution.
also take up considerable amount of space. Therefore it will take less than 5.00 L of water to make the 5.00 L solution.