Hello!
The answer is:
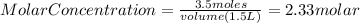
Why?
Since there is not information about the solute but only its mass, we need to assume that we are calculating the molar concentration of a solution or molarity. So, need to use the following formula:

Now, we know that the mass of the solute is equal 3.5 moles and the volume is equal to 1500 mL or 1.5L
Then, substituting into the equation, we have:
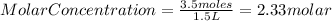
Have a nice day!