Answer:
Benzoic acid is the stronger acid
Step-by-step explanation:
Weak acids do not dissociate completely in the solution. They exists in equilibrium with their respective ions in the solution.
The extent of dissociation of the acid furnising hydrogen ions can be determined by using dissociation constant of acid (
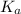 ).
).
Thus for a weak acid, HA
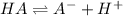
The
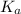 is:
is:
![K_a= ([A^-][H^+])/([HA])](https://img.qammunity.org/2020/formulas/chemistry/college/ybw04fs24hd8q1jpfs8j49q9krszb8a1ls.png)
The more the
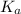 , the more the acid dissociates, the more the stronger is the acid.
, the more the acid dissociates, the more the stronger is the acid.
Also,
 is defined as the negative logarithm of
is defined as the negative logarithm of
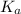 .
.
So, more the
 , less is the
, less is the
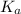 and vice versa
and vice versa
All can be summed up as:
The less the value of
 , the more the
, the more the
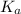 is and the more the acid dissociates and the more the stronger is the acid.
is and the more the acid dissociates and the more the stronger is the acid.
Given,
 of acetic acid = 54.7
of acetic acid = 54.7
 of benzoic acid = 54.2
of benzoic acid = 54.2
 of benzoic acid <
of benzoic acid <
 of acetic acid
of acetic acid
So, benzoic acid is the stronger acid.