Answer: The value of change in internal energy of the system is, 40 J.
Explanation : Given,
Heat absorb from the surroundings = 12 J
Work done on the system = 28 J
First law of thermodynamic : It is a law of conservation of energy in which the total mass and the energy of an isolated system remains constant.
As per first law of thermodynamic,
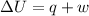
where,
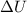 = internal energy = ?
= internal energy = ?
q = heat absorb from the surroundings
w = work done on the system
Now put all the given values in this formula, we get the change in internal energy of the system.
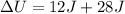
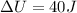
Therefore, the value of change in internal energy of the system is, 40J.