Answer:
 is not likely to have ionic bonds
is not likely to have ionic bonds
Step-by-step explanation:
Ionic bond is formed between a metal and a non metal.
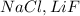 and
and
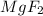 are examples in which we see metal bonded with a non-metal.
are examples in which we see metal bonded with a non-metal.
Na, Li, Mg are all metals and Cl, F are non metals.
 contains only non metals. Covalent bond is the bond present among non metallic compounds.
contains only non metals. Covalent bond is the bond present among non metallic compounds.
We can identify a bond as an ionic bond or a covalent bond based on the electronegativity values of the atoms present in it.
For example
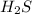
Hydrogen --> electronegativity value is 2.1
Sulfur --> electronegativity value is 2.5
∆E, the electronegativity difference is 2.5 - 2.1 = 0.4
So, It is a non polar covalent bond present in
Examples :
The electronegative values of H is 2.1
F is 4.0
O is 3.5
N is 3.0
∆E value of HF Hydrogen bond is
4.0 - 2.1 = 1.9
∆E value of H2O Hydrogen bond is
3.5 - 2.1 = 1.4
∆E value of NH3 Hydrogen bond is
3.0 - 2.1 = 0.9
∆E value between 0.5 to 1.7 is a polar covalent bond
∆E value btween 0 to 0.4 is a non polar covalent bond
∆E above 1.7 is an ionic bond