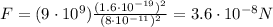Answer:
Step-by-step explanation:
The electrostatic force between the proton and the electron is given by:
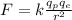
where
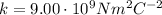 is the Coulomb constant
is the Coulomb constant
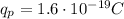 is the magnitude of the charge of the proton
is the magnitude of the charge of the proton
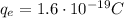 is the magnitude of the charge of the electron
is the magnitude of the charge of the electron
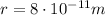 is the distance between the proton and the electrons
is the distance between the proton and the electrons
Substituting the values into the formula, we find