Answer : The specific latent heat of fusion of ice is, 187.608 J/g
Explanation :
Formula used :
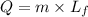
where,
Q = heat supply = 863 KJ = 863000 J
conversion used : (1 KJ = 1000 J)
m = mass of the substance = 4.6 Kg = 4600 g
conversion used : (1 Kg = 1000 g)
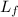 = specific latent heat of fusion = ?
= specific latent heat of fusion = ?
Now put all the given values in the above formula, we get the specific latent heat of fusion of ice.
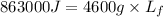
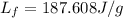
Therefore, the specific latent heat of fusion of ice is, 187.608 J/g