Answer:
D. A syringe pulls in air as the plunger is pulled back.
Step-by-step explanation:
As per Charles’s law
At constant pressure for a given amount of a gas,
Volume is directly proportional to its temperature.
Directly proportional means when one increases the other one too will increase. That is when volume increases temperature too will increase. When volume decreases, temperature too will show a decrease.
Option A tells us that the volume of the hot air balloon increase due to the rise(increase) in temperature as the balloon is heated
Thus the expression is
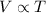
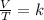 where k is a constant
where k is a constant
When there is a change in the volume and temperature the expression will be
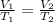
where
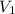 and
and
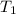 are the initial volume and initial temperature and
are the initial volume and initial temperature and
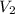 and
and
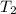 are the final volume and temperature.
are the final volume and temperature.