Answer:
A. electronegativity < 1.0
Step-by-step explanation:
Hydrogen --> electronegativity value is 2.1
Sulfur --> electronegativity value is 2.5
∆E, the electronegativity difference is 2.5 - 2.1 = 0.4
So, It is a non polar covalent bond present in
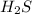
Examples :
The electronegative values of H is 2.1
F is 4.0
O is 3.5
N is 3.0
∆E value of HF Hydrogen bond is
4.0 - 2.1 = 1.9
∆E value of H2O Hydrogen bond is
3.5 - 2.1 = 1.4
∆E value of NH3 Hydrogen bond is
3.0 - 2.1 = 0.9
∆E value between 0.5 to 1.7 is a polar covalent bond
∆E value btween 0 to 0.4 is a non polar covalent bond
∆E above 1.7 is an ionic bond