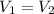Answer:
Not Changed
Step-by-step explanation:
To know what happened with the volume you need to know the Ideal gas Law
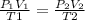
This law is a combination of the other four laws: Boyles's, Charles's, Avogadro's, and Guy-Lussac's.
The initial state is represented by P1, V1, T1 and the final by P2, V2, T2.
In this case:
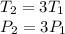
Replacing on the equation
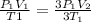
If we clear from the equation V2
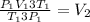
Then cancel both P1 and T1
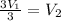
You will found that