Answer:
a)
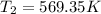
b)Work done per kg of air=196.84 KJ/Kg
Step-by-step explanation:
Given:
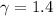 for air.
for air.
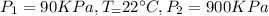
We know that
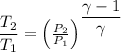
So
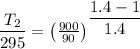
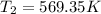
(a)
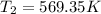
(b)Work for adiabatic process
W=
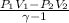
We know that PV=mRT for ideal gas.
W=
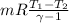
Now by putting values
work per kg of air=
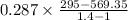
Work w=-196.84 KJ/Kg (Negative sign indicate work given to input.)
So work done per kg of air=196.84 KJ/Kg