Solution:
Given:
mass of air, m = 0.50 Kg
 = 25°C = 273+25 = 298 K
= 25°C = 273+25 = 298 K
 = 100°C = 273+100 = 373 K
= 100°C = 273+100 = 373 K
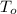 = 200°C = 273+100 = 473 K
= 200°C = 273+100 = 473 K
Solution:
Formulae used:
ΔQ = mCΔT (1)
ΔS =
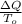 (2)
(2)
where,
ΔQ = change in heat transfer
ΔS = chane in entropy
C = specific heat
ΔT = change in system temperature
Using eqn (1)
ΔQ =
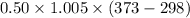 = 36.687 kJ
= 36.687 kJ
Now, for entropy generation, using eqn (2)
ΔS =
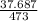 = 0.0796 kJ
= 0.0796 kJ