Answer:
5000J
Step-by-step explanation:
Given in the question that
Heat added to the coffee cup is, ΔS = 20 J/K
The temperature of the coffee, T = 250 K
Now, using the formula for the entropy change
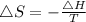 ...........(1)
...........(1)
Where,
ΔS is the entropy change
ΔH is the enthalpy change
T is the temperature of the system
substituting the values in the equation (1)
we get
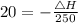
ΔH=250×20
ΔH=5000 J