Answer:
Decrease the volume or increase the number of moles by a factor of 37.
Step-by-step explanation:
If the contents are a gas,
pV = nRT
If T is constant
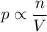
There are two ways to increase the pressure inside the container.
1. Decrease the volume
If you decrease the volume by a factor of 37, the pressure will increase by the same factor.
2 Increase the number of moles
If you add 36 times the number of moles, you will have 37n moles. Increasing the amount of gas by a factor of 37 increases the pressure by the same factor.