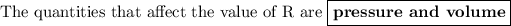Answer:
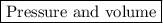
Step-by-step explanation:
The measure of R is always the same, but the numbers may differ depending on the units you use.
For example, in SI units, R = 8.314 Pa·m³K⁻¹mol
If your measurement uses different units, you must either convert your units to SI or use a value of R consistent with your units.
If you use bars and litres, R = 0.083 14 bar·L·K⁻¹mol⁻¹.
If you use kilopascals and litres, R = 8.314 kPa·L·K⁻¹mol
If you use atmospheres and litres, R = 0.082 06 L·atm·K⁻¹mol⁻¹.
If you use Torr and cubic centimetres, R = 62 368 Torr·cm³ K⁻¹mol⁻¹.
The only units that don't change are "K⁻¹mol⁻¹".