First of all, as you seen the gases are noble which means that will not react with each other and in this case each gas create individual pressure.
P
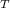 = total pressure
= total pressure
P
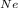 = pressure of neon
= pressure of neon
P
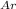 = pressure of argon
= pressure of argon
P
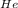 = pressure of helium {which is required}
= pressure of helium {which is required}
P
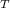 = P
= P
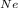 + P
+ P
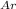 + P
+ P
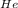
1.25 = 0.68 + 0.35 + P
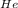
P
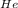 = 1.25 - [0.68 + 0.35] = 0.22 atm
= 1.25 - [0.68 + 0.35] = 0.22 atm